These days, knowing a patient’s genetic make-up means that we can diagnose many diseases and determine certain hereditary predispositions. The potential is huge. Some experts predict that in the future DNA testing will become standard medical procedure. But we must beware, as danger lurks along this path.
BRCA1 and BRCA2. These mysterious acronyms refer to two genes. A mutation in these genes can reduce the body’s natural defence and increase the risk of developing breast and ovarian cancer. These genes prompted the highly public decision made by American actress Angelina Jolie in 2013 to have her breasts and ovaries removed following a DNA test. The test found that she had around a 70% chance of developing breast cancer and about 40% of developing ovarian cancer.
Her decision to have the operation based on risk factors, and her courage to communicate openly about it in the media had tremendous consequences. In the month following her procedure, the number of screening tests for BRCA1 and BRCA2 genes increased by about 50% in the United States. Identical figures were seen at Lausanne University Hospital (CHUV) and in Switzerland. More importantly, Angelina Jolie’s case showed the general public the potential of gene testing in medical diagnosis and treatments. And it foretells of a future in which doctors can study patients’ genetic make-up to provide care before illness even develops.
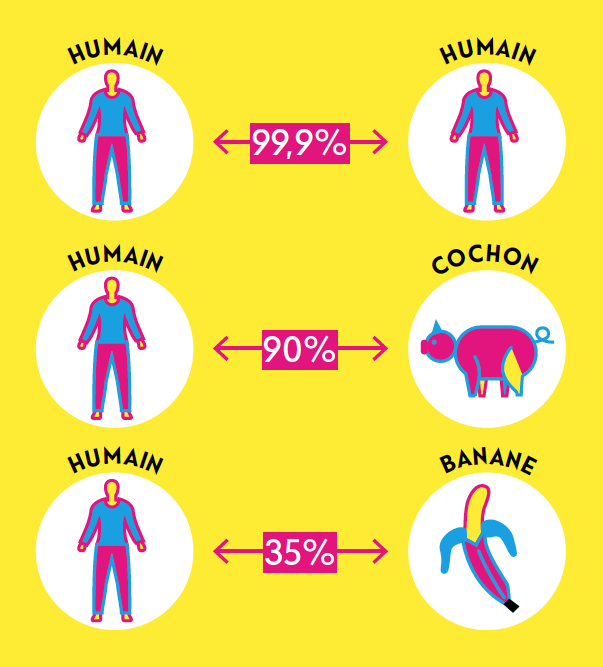
Today, DNA testing is mainly used to diagnose monogenic disorders (or rare diseases). These disorders are caused by mutations – experts refer to them as genetic variants – in a single gene out of the 20,000 in the human body. Cystic fibrosis and Huntington’s disease are examples of these diseases. In all, about 6,000 have been identified. They affect roughly 500,000 people in Switzerland.
Then there are polygenic diseases. These conditions are caused by variants in several genes. External factors such as pollution, diet and lifestyle also influence the development of this category of diseases, which includes obesity, diabetes, asthma, allergies and coronary heart disease. In these cases, DNA testing is only used to indicate the chances of developing a disease in their lifetime.
With cancer, it’s a bit different. Most cases occur sporadically over the course of a patient’s life, and doctors don’t always know exactly why. Environmental factors such as smoking and exposure to chemicals also play a role in the development of cancer. But a minority of forms of cancer – around 5% to 10% – is due to hereditary predisposition, or pathogenic variants in genes from birth. These variants considerably increase the risk of developing certain types of cancer, as was the case with Angelina Jolie.

DNA is tested using genome sequencing. This technique involves using a blood or saliva sample to create an exact image of our DNA, either part of the genome or the entire genome with its 6 billion nucleotides. What sequencing does concretely is deliver a very long sequence of the letters A, C, G and T. Some parts of this sequence make up a gene. Researchers and doctors are mainly interested in these parts to detect potential abnormalities.
DNA sequencing is a relatively young field. In 2003, researchers from the Human Genome Project, an international research project, sequenced a complete human genome for the first time. At the time, the process had taken several years. And now?
“A whole genome can be sequenced in about 24 hours. But analysing the genome and genes to deliver a medical diagnosis takes a few weeks,” says Professor Jacques Fellay, Director of the CHUV’s Precision Medicine Unit.
The technology continues to develop fast. For example, a hospital in San Diego (United States) recently implemented a system that combines sequencing with artificial intelligence to produce a diagnosis in one day.
Artificial intelligence plays a key role in detecting variants. As each genome contains a huge amount of data, it is not feasible to analyse them individually. Instead, algorithms are used to compare them with other genomes, enabling analysts to draw meaningful conclusions and make a diagnosis. That means that one genome can be compared with thousands of other genomes stored anonymously in databases. For example, CHUV professionals compare their sequences carried out for diagnostic purposes with data from more than 100,000 healthy individuals in the American gnomAD database, explains Professor Andrea Superti-Furga, Director of the CHUV’s Service of Genetic Medicine.
“The larger the comparative database, the more precise the results will be,” Professor Fellay says. For example, the American National Institutes of Health (NIH), the nation’s medical research agency, aims to collect genetic information on one million citizens by 2024. In Switzerland, the CHUV set up a biobank in 2013 which currently stores around 30,000 blood samples. But the samples have not yet undergone DNA testing. To increase their number, in early April the CHUV updated the general patient consent form, which dated from 2013. In the new version, patients can consent to the use of genetic information from a given sample for research.


For now, DNA sequencing is mainly used at hospitals for diagnosis. At the Service of Genetic Medicine at the CHUV, several hundred sequences are carried out each year, followed by a targeted bioinformatics analysis, in which doctors focus on certain genes.
In addition to these targeted analyses, since 2018 60 patients have had their genome completely sequenced, where doctors look at all genes. How do they decide whether to use targeted or whole genome sequencing? Whole genome sequencing is used to detect serious pathologies that could not be diagnosed conventionally or via targeted gene sequencing. Another factor is financial. Targeted sequencing is reimbursed by health insurance, but whole genome sequencing is not. Insurance companies have not yet recognised the effectiveness of this technique. Professor Superti-Furga has agreed to cover the costs of whole genome sequencing for the 60 individuals, amounting to several thousand Swiss francs, as part of a project to develop this method of diagnosis.
The reliability of targeted or whole genome sequencing depends on the number of genes involved in developing the disease. For example, around 50 genes are associated with kidney diseases, whereas about 1,500 genes are involved in cognitive development disorders. Doctors therefore do not yet fully understand the relationship between certain genes and certain diseases, but scientific advances are moving fast.
“Between 5,000 and 6,000 genes are now clearly associated with certain diseases. And new discoveries are made every week. That means that individual genome sequencing is likely to yield new results in a few years’ time,” Professor Superti-Furga says.
Even for monogenic diseases, doctors are far from understanding all the mechanisms involved around the genes. This was illustrated with the case of Bruno Aerni, who suffers from a serious neurodegenerative disease. Doctors were first able to establish a link with a gene called GNAO1. But this was not enough to make a full diagnosis. After several unsuccessful attempts with targeted gene sequencing, his whole genome was sequenced and compared with countless data. And that finally provided the answer: a mutation in a single “letter” (nucleotide) preceding the GNAO1 gene in the genome. This abnormality has only been observed in hundred other cases worldwide. “Even though we often know which genome sequence to look for, we don’t always have the ‘glasses’ to read the genes. We don’t yet fully understand how certain parts of DNA act on the body or whether they cause disease,” Professor Superti-Furga adds. “But genome sequencing is offering us some valuable insight.”


Many researchers and doctors are convinced that genome sequencing will lead to a better understanding of many diseases. For example, the NIH invests half of its $42 billion annual budget in genomics research. Topping the list is cancer. One of the most comprehensive projects in cancer research has recently been completed under the Pan-Cancer Analysis of Whole Genomes, or PCAWG, Consortium. Launched in 2016, the project involved 1,300 researchers from around the world. Nearly 3,000 genomes covering 38 tumour types were sequenced. “We wanted to better understand which mutations within a tumour make it malignant”, says Moritz Gerstung from the European Bioinformatics Institute, one of the project leaders.
Certain tumours, such as those that cause skin cancer, develop up to 100,000 mutations, 20% of which make the tumour dangerous for the body. “If we better understand the harmful potential of mutations and when they occur in the DNA of a tumour cell, doctors can intervene much earlier,” he adds. Gerstung believes that the first real applications in oncology could be available within the next few years.
A research project has recently been launched at the CHUV to more accurately select cancer patients who could be treated with immunotherapy. This promising treatment uses the patient’s immune defences to attack and destroy cancer cells. As most patients do not respond to treatment, the project aims to better understand which genetic predispositions are compatible with immunotherapy.
Another application being studied is in pharmacogenetics. This field looks for genetic variants in the body that cause side effects with certain drugs. For example, our genes affect liver enzymes, which are essential for breaking down drugs. When a genetic mutation prevents enzymes from working properly, the drug can build up in the body and cause serious side effects.
Recognised as the most innovative company in its field in Europe, the Vaud-based company Gene Predictis has developed a tool based on a genetic test and an algorithm to measure the risk of thrombosis linked to the use of the contraceptive pill. Another product helps doctors to adjust the dosage of certain drugs, such as painkillers, based on the patient’s genome. The company’s CEO Goranka Tanackovic says that several hundred doctors in Switzerland are already using these solutions. However, applications in pharmacogenetics remain marginal, as Professor Superti-Furga points out, “In clinical practice, genomics plays a role in only a limited number of situations, such as measuring doses of anticoagulants or choosing a contraceptive.”
Genome sequencing can produce valuable information about a person. When this technique is used to make a medical diagnosis, it can reveal variants that could potentially cause other diseases or predispositions. The more genes you analyse, the more likely you are of uncovering something. Should a hereditary predisposition, i.e. a genetic variant, be disclosed to the patient? Even if no treatment is available? Answering these questions and guiding patients through the sequencing process is one facet of the job for Marie Met-Domestici, who is one of four genetic counsellors at the Service for Genetic Medicine at the CHUV. Before the DNA test, she has to explain to patients what is at stake. After the analysis, she helps in communicating the results to them.
Ms Met-Domestici mainly cares for patients suspected of having a hereditary predisposition to cancer. For women with breast cancer, a genetic analysis may show that she is predisposed to the disease, especially when it involves a mutation in the BRCA genes. This finding could lead to adapting her treatment, such as more extensive surgery to reduce the risk of a second cancer. For the genetic counsellor, this raises important questions: “Results of the analysis should be integrated appropriately into the patient’s care pathway. For example, a young patient who has already been diagnosed realises how hereditary predisposition could affect his or her family. We may also find predispositions to other types of cancer than the form that was the initial subject of analysis. So that needs to be clearly discussed beforehand.”
Marie Met-Domestici clarifies that the role of genetic counsellors is to encourage each patient to decide whether they want to initiate a genetic analysis. She feels that as more and more people gain access to genetic analysis, proper counselling is still needed before and after the procedure to integrate the emotional impact of the process.

Medicine has undeniably advanced thanks to DNA sequencing. “Only about a thousand monogenic diseases were known as of the 2000s. Through genome sequencing, we know of almost six times as many today,” recalls Professor Superti-Furga. Caused by several genes and external factors, polygenic diseases are more complicated to understand. Professor Fellay mainly sees the potential in developing more preventive care: “Of course, lifestyle and environment play a very important role in many diseases. But knowing everyone’s genome will also help, for example, in adapting screening tests. A woman could have a mammogram earlier or later depending on her genetic predisposition. In the end, every disease is partly genetic.” But that leaves many unanswered questions. “Is it really a blessing to know that you’re predisposed to develop a certain disease if there is no prevention or treatment available?” Professor Superti-Furga points out. “Precision medicine works well for monogenic diseases, but for polygenic diseases, prevention should focus on public health measures such as diet, physical activity, smoking prevention campaigns and so on.”
The number of nucleotides contained in our DNA.
The number of genes that make up our genetic material.
Amount of DNA that each human cell contains.